
Components: SARS-CoV-2 Antigen Test Cassette, Sample Tube, and Disposable Sterile Swab
Usage: Qualitative immunoassay, detection of nucleocapsid protein antigen from SARS-CoV-2 virus
Applicable crowd:
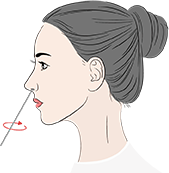
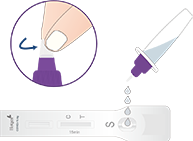
Non-prescription home use with self-collected anterior nasal (nares) swab samples
For individuals aged 14 years or older
For individuals aged two years or older, please collect anterior nasal (nares) swab samples with
assistance from adults
Within the first 7 days of Covid-19 symptoms onset
Specifications: 1Test, 2Tests, 5Tests, 10Tests, 20Tests, 40Tests
Sample type: Human anterior nasal (nares) swabs
As of XX, the expiration date of the Hotgen™ COVID-19 Antigen Home Test has been extended by X months. Please click here to check the new expiration date using the lot number printed on the test kit box. For the most current expiration dates of this test, please refer to: http://www.fda.gov/covid-tests








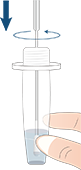





As shown in Fig. 1, if both the Control (C) line and the Test (T) line are visible, the test is positive. Any faint visible red/purple test (T) line with the control line (C) should be read as positive.
Note: You do not need to perform repeat testing if you have a positive result at any time.

As shown in Fig. 1, if both the Control (C) line and the Test (T) line are visible, the test is positive. Any faint visible red/purple test (T) line with the control line (C) should be read as positive.
Note: You do not need to perform repeat testing if you have a positive result at any time.

As shown in Fig. 1, if both the Control (C) line and the Test (T) line are visible, the test is positive. Any faint visible red/purple test (T) line with the control line (C) should be read as positive.
Note: You do not need to perform repeat testing if you have a positive result at any time.



For questions or to report a problem, please call 1-800-966-2919 (available Monday through Friday: 9 a.m. to 5 p.m. PST) or cs@hotgen.info.
The MedWatch reporting system can also be used




This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA.
This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.